Hydatothrips adolfifriderici Karny, 1913
Sericothripinae, Thripidae, Terebrantia, Thysanoptera
Figures
Fig. 1: 8-segmented antenna, segments III and IV with forked sense cone, terminal segments VI-VIII
Fig. 2: Head dorsal with ocellar triangle
Fig. 3: Pronotum
Fig. 4: Meso- and metanotum
Fig. 5: Meso- and metasternum with meso- and metasternal furca
Fig. 6: Fore wing, fore wing distal region
Fig. 7: Tergites II-V
Fig. 8: Tergites VI-VIII
Fig. 9: Tergites VI and VIII with posteromarginal comb
Fig. 10: Tergites VIII-XI
Fig. 11: Adult female
Introduction and recognition
Hydatothrips adolfifriderici causes damage on leaves of many legumes, particularly cowpea and soya bean crops. Female macropterous; body strikingly bicolored, mainly brown with abdominal segments 4 & 5 yellow, also anterior region of pronotum and posterior region of head; tibiae and tarsi yellow or pale, also antennal segments I-III and basal half of IV and V; fore wings light brown with sub-basal pale band. Antennae 8-segmented; segments III and IV with sense cone long and forked, VI with base of sense cone elongate (Fig. 1). Head wider than long; postocular region with transverse reticulation; ocellar triangle with transverse lines of sculpture; 3 pairs of ocellar setae, pair III on anterior margins of triangle; 3 pairs of small postocular setae; with a distinct occipital ridge (Fig. 2). Pronotum broadly reticulate on anterior half and laterally, but with pronotal blotch bearing closely spaced transverse lines on posterior half, pronotal blotch dark and not emarginate posteriorly, 1 pair of moderately long setae near posterior angles (Fig. 3).
Meso- and metafurca with stout median spinula.
Metanotum median area with sculptured lines transverse at anterior, but forming irregular longitudinal reticulations on posterior half; median setae varying in position; campaniform sensilla absent (Fig. 4); metasternum with distinctive V-shaped anterior margin (Fig. 5). Mid- and hind tarsi 2-segmented. Fore wing first vein distict from costal vein and with a continuous row of setae; second vein without setae (Fig. 6). Lateral thirds of tergites I-VIII with many rows of fine and regular microtrichia; tergites I-VI with posteromarginal comb of microtrichia present laterally, incomplete medially (Fig. 7); tergites VII and VIII with complete comb of long and regular microtrichia on broadly triangular bases (Fig. 8-10); tergites I-VIII with median pair of setae not similarly placed, nor of similar size on all tergites, median setal pair longer than distance between their bases. Sternites without discal setae but fully covered with rows of microtrichia; posterior margins with comb of long microtrichia between the marginal setae; sternite VII posteromarginal seta S1 arising in front of margin.
Male similar to female; with a specialised cuticular area spanning the anteriorly on each of sternites VI & VII; sternites without glandular areas.
Taxonomic identity
Species
Hydatothrips adolfifriderici Karny, 1913
Taxonomic history
Sericothrips bedfordi Priesner, 1964
Sericothrips adolfifriderici Priesner, 1949
Sericothrips occipitalis Hood, 1917
Common name
-
Present taxonomic position
Family: Thripidae Stephens, 1829
Subfamily: Sericothripinae Karny, 1921
Genus: Hydatothrips Karny, 1913
Genus description
The genus Hydatothrips Karny, 1913
About 40 species are included in this genus from various parts of the tropics. Hydatothrips is a genus of the subfamily Sericothripinae, closely related to members of the both genera of Neohydatothrips and Sericothrips. These thrips all have numerous closely spaced rows of microtrichia on the lateral thirds of the tergites. Most of them have a distinctive colored and/or sculptured area on the pronotum, the pronotal blotch. In almost all of the species the sense cone on the sixth antennal segment has a remarkably elongate and narrow base, and the fore wing first vein has a complete row of setae but the second vein has no or only 2 setae. Bhatti (1973) has given identification keys to distinguish the various genera involved. All of the species of Hydatothrips have the anterior margin of the metasternum deeply recessed to form a V-shaped sclerite and most of them are strikingly bicolored with banded wings.
Species description
Typical character states of Hydatothrips adolfifriderici
Coloration and body sculpture
Body color: distinctively bicolored
Surface of head, pronotum and fore legs: without obvious or with weakly reticulate sculpture
Antennae
Form of sense cones on antennal segments III and IV: emergent and forked on segments III and IV
Number of antennal segments: 8
Head
Distance between bases of ocellar setae III: greater than width of first ocellus
Ocelli: present
Ocellar setae I: present
Ocellar setae III: arising on anterior margin of, or in front of ocellar triangle
Prothorax
Pronotal blotch or internal apodeme: present
Pronotal blotch: dark and not emarginate posteriorly
Pronotum shape: broadly rectangular
Pronotum surface: anterior with closely reticulate and/or posterior with more striate sculpture
Mesothorax
Mesosternal furca: with median spinula
Metathorax
Metanotum with dominant sculptured triangle medially: absent
Metasternal furca: with spinula
Metasternum anterior margin: with deeply V-shaped apodeme
Sculpture of metanotum median area: transverse at anterior, but irregular longitudinal or equiangular reticulations on posterior half
Shape of metathoracic furca: transverse, V-shaped
Metascutellum: without microtrichia
Wings
Fore and hind wings: present, more than half as long as abdomen (macropterous)
Fore- and hind wing surface: covered with microtrichia
Fringe cilia arising: from sockets
Fore wing surface: not reticulate
Fore wing veins: present
Apex of fore wing: with prominent terminal setae
Fore wing costal fringe cilia: arising at anterior margin of wing
Fore wing first vein: distinct from costal vein
Fore wing first vein setal row: complete, with setae closely and uniformly spaced
Fore wing second vein setal row: with no setae
Fore wing shape: mainly parallel sided or margins run continuously towards each other
Shape of fore wing apex: with mainly posterior margin curved to join anterior margin
Fore wings: uniformly dark or shaded, but with base or sub-base pale
Legs
Mid and hind tarsi: with two segments
Abdomen
Sternites V and VI microtrichia: extending fully across discal area
Sternite VII median posteromarginal setae S1: arising in front of posterior margin
Microtrichia on tergites II to VII: only on lateral thirds, median area smooth or with sparse microtrichia
Surface of lateral thirds of abdominal tergites: with many regular rows of fine microtrichia
Median setal pairs on tergites II to VII: dissimilar in size and position
Tergites IV and V median setal pair: longer than distance between their bases
Markings on tergites IV to VI: with shaded areas medially
Tergite VIII ctenidia: without paired ctenidia laterally, sometimes with irregular microtrichia
Tergite VIII posteromarginal comb of microtrichia: present and complete medially
Tergite VIII shape of posteromarginal microtrichia: long, slender and regular on broadly triangular bases
Tergite X: not tubular, longitudinally incomplete

Similar or related species
Hydatothrips adolfifriderici is closely related to Neohydatothrips samayunkur and Sericothrips sativus. Compared to these both species, Hydatothrips adolfifriderici has a metasternum with anterior margin deeply U-shaped and median V-shaped apodeme, a dark pronotal blotch that is not emarginate posteriorly, and a metanotal median area sculptured lines are transverse at anterior, but forming irregular longitudinal reticulations on posterior half. Neohydatothrips samayunkur as well as Sericothrips sativus have the metasternum anterior margin almost transverse. In Neohydatothrips samayunkur the dark pronotal blotch is deeply emarginate posteriorly, and the metanotal median area sculptured lines are also transverse at anterior, but longitudinal and parallel on posterior half. In Sericothrips sativus the pronotal blotch is pale, and the metanotal median area sculptured lines are transverse striate. Hydatothrips adolfifriderici as well as Neohydatothrips samayunkur have no microtrichia on metascutellum, dense microtrichia on only on lateral thirds of tergites I-VIII and median area smooth or with sparse microtrichia, tergites I-VI posteromarginal comb of microtrichia is present laterally but incomplete medially, median setae are short on tergites I-IV and longer on tergites V-VIII, and the median setal pair is longer than the distance betwen their bases. Whereas Sericothrips sativus has a metascutellum covered with bands of microtrichia medially, tergites I-VIII are completely covered with microtrichia, a complete posteromarginal comb of microtrichia on tergites I-VI, and tergal median setae similarly placed and of equal size.
Biology
Life history
As with other thrips species the life cycle from egg to adult is dependent on temperature. The full cycle can take less than one week to over a month and adults may live for more than one month producing several generations in one year depending on seasonal weather (Lewis 1973).
Host plants
Mostly on leaves of Fabaceae.
Crops: African nightshade, alfalfa, amaranth, arrowroot, beans (broad bean, common bean, cowpea, French bean, hyacinth bean, snow pea, soyabean, zombi pea), brocolli, cashew, chillies, eggplant, green gram, kale, leek, maize, mango, okra, onion, passion fruit, potato, pumpkin, red gram, sweet potatao, tomato, watermelon.
Weeds: Bidens pilosa, Clerodendron myricoides, Datura suaveolens, Galinsoga parviflora, Nycandra physalodes, Ocimum sp., Senna bicapsularis, Senna occidentalis, Sesbania sesban, Stachytarpheta jamaicensis, Tagetes minuta, Tephrosia villosa ssp. ehrenbergiana.
Vector capacity
None identified, but possible mechanical distribution of phytopathogenic fungi and bacteria.
Damage and symptoms
Hydatothrips adolfifriderici was one among the key pest thrips of French beans in Kenya along with Frankliniella occidentalis, Frankliniella schultzei and Megalurothips sjostedti (Nyasani et al. 2012) and 68 - 70% yield losses was observed due the thrips complex infesting French beans.
Detection and control strategies
Adults of Hydatothrips adolfifriderici were attracted to yellow coloured sticky traps, while they were not attracted to blue coloured sticky traps (Muvea 2011). Captures of Hydatothrips adolfifriderici on yellow sticky traps are significantly enhanced by addition of the kairomonal attractant LUREM-TR (Nielsen et al. 2010; Muvea 2011). In Kenya, populations of the African bean flower thrips (Megalurothips sjostedti) and Hydatothrips adolfifriderici on cowpea buds were almost halved by intercropping the cowpea with sorghum and maize (Dissemond & Hindorf 1990).
Sprays on cowpea with 2.5% to 10% aqueous Neem Seed Kernal Extract effectively repelled the adults of Hydatothrips adolfifriderici (=Sericothrips occipitalis) and Megalurothips sjostedti. This resulted in more than 60% increase in pod yield (Dreyer 1986). Application of entomopathogenic fungi, Metarhizium anisopliae isolate ICIPE69 effectively reduced the numbers of Hydatothrips adolfifriderici and was comparable to application of alpha-cypermethrin (Muvea 2011). The eulophid parasitoid, Cerenisus menes did not parasitize both first and second instars of Hydatothrips adolfifriderici (Kwamboka et al., unpublished data).
Additional notes
Feeding and breeding on leaves.
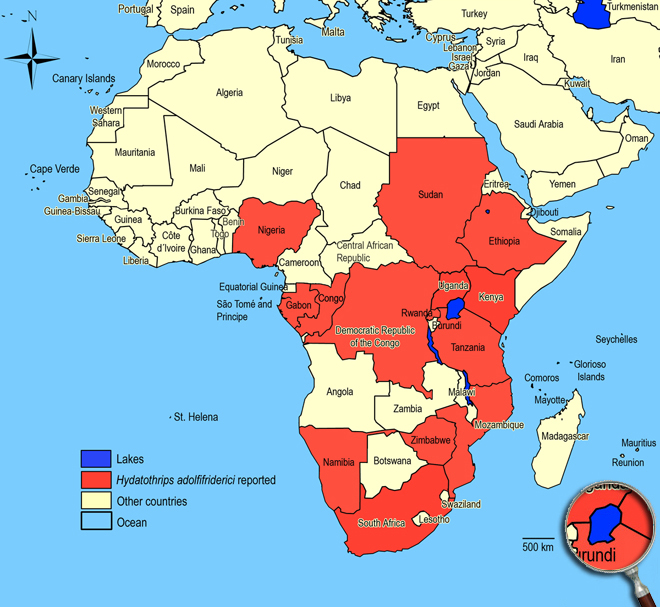
Biogeography
Afrotropical Region. Congo (Nioka, Kivu),
Ethiopia (Akaki river - Addis Ababa),
Gabon,
Kenya (Mbita),
Mozambique, (Chinanganine),
Namibia (Kaokoland),
Nigeria (Ibadan),
Rwanda (Lake Bolero),
South Africa (Limpopo: Pietersburg, Zebediela; Gauteng: Pretoria; KwaZulu-Natal: Hlabisa, St. Lucia, St. Lucia Lake, Port Shepstone; North West: Rustenburg),
Sudan (Khartoum, Wad Medani, Rejaf),
Tanzania (Ol Joro),
Uganda, Zimbabwe (Harare).
African countries where Hydatothrips adolfifriderici has been reported
Occurence of Hydatothrips adolfifriderici in East Africa
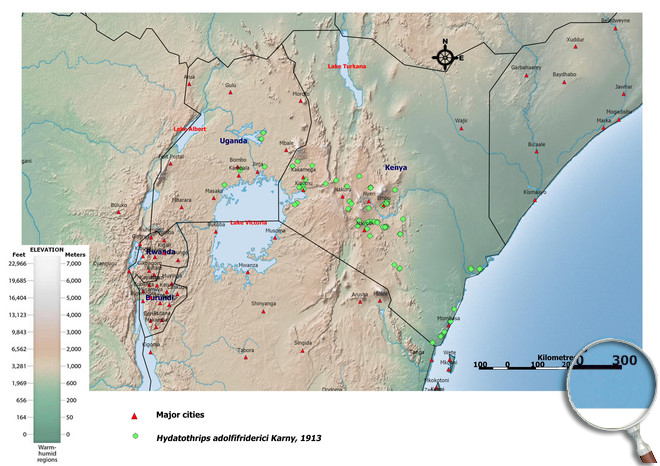
Please click here for survey sites of all observed thrips species of Kenya, Tanzania and Uganda.
Click here for locations of Hydatothrips adolfifriderici in parts of East Africa.

Bibliography
Bhatti JS (1973). A preliminary revision of Sericothrips Haliday, sensu lat., and related genera, with a revised concept of the tribe Sericothripini (Thysanoptera: Thripidae). Oriental Insects. 7 (3): 403-449
Bournier A (1970). Thysanoptères du Gabon. Extrait de la Revue Biologia Gabonica. 6 (2): 151-168
Dissemond A & Hindorf H (1990). Influence of sorghu/maize/cowpea intercropping on the insect situation at Mbita/Kenya. Journal of Applied Entomology. 109: 144-150
Dreyer M (1986). Untersuchungen zur Wirksamkeit von Wasserextrakten und anderen Produkten aus Niemsamen gegen Schädlinge an Gemüse- und Freilandkulturen in Togo. Diss. Univ. Giessen
Faure JC (1956). Thysanoptera from papyrus in the Sudan. Journal of the Entomological Society of Southern Africa. 19 (1): 100-117
Faure JC (1958). South African Thysanoptera - 9. Journal of the Entomological Society of Southern Africa. 21 (2): 354-375
Faure JC (1962). Thysanoptera of Africa - 8. Revue de Zoologie et de Botanique Africaines. 66 (1-2): 165-183
Hartwig EK (1952). Taxonomic studies of South African Thysanoptera, including genitalia, statistics and a revision of Trybom's types. Entomology Memoirs. 2 (11): 340-499
Hood JD (1917). A new Sericothrips (Thysanoptera) from Africa. Bulletin of the Brooklyn Entomological Society. 12 (2): 32-34
Jacot-Guillarmod CF (1937). Ten new species of Thysanoptera and a catalogue of the known South African forms. Publications of the University of Pretoria, Serie 2, Natural Science. 3: 1-63
Karny H (1913). Thysanoptera. Wissenschaftliche Ergebnisse der Deutschen Zentral-Afrika Expedition 1907-1908, unter Führung Adolf Friedrichs, Herzogs zu Mecklenburg. 4 (Zoologie, 2): 281-282
Kudo I (1997). Malaysian Hydatothrips with some species from neighboring areas (Thysanoptera, Terebrantia, Thripidae). Japanese Journal of Systematic Entomology. 3: 325-365
Lewis T (1973). Thrips: their biology, ecology and economic importance. Academic Press Inc., London Ltd., 349 pp
Moritz G (2006). Thripse. Pflanzensaftsaugende Insekten, Bd. 1, (1. Auflage). Westarp, Hohenwarsleben, 384 pp. ISBN-13: 978 3 89432 891 7
Moritz G, Morris DC & Mound LA (2001). ThripsID - Pest thrips of the world. ACIAR and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1 86320 296 X
Moritz G, Mound LA, Morris DC & Goldarazena A (2004). Pest thrips of the world - an identification and information system using molecular and microscopical methods. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN 1 86499 781 8
Moritz G, O'Donnell C & Parrella M (2009). Pest thrips of North America. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN-13: 978 1 86499 940 2
Moulton D (1930). Thysanoptera from Africa. Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 10) 5: 194-207
Mound LA & Kibby G (1998). Thysanoptera: An identification guide, (2nd edition). CAB International, Wallingford and New York, 70 pp
Mound LA & Marullo R (1996). The thrips of Central and South America: An introduction (Insecta: Thysanoptera). Memoirs on Entomology, International, Vol. 6. Associated Publishers, Gainsville, 487 pp
Muvea AM (2011). The Potential of Coloured Sticky Traps with Kairomonal Attractants (LUREM-TR) in Management of Thrips on Tomato and French beans. Unpublished thesis, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya, 107 pp
Nielsen MC, Worner S, Chapman B, de Kogel W-J, Perry N, Sansom C, Murai T, Muvea A, Subramanian S, Davidson M & Teulon D (2010). Optimising the use of allelochemicals for thrips pest management. Proceedings of the International Society of Chemical Ecology Conference, 26th Annual Meeting, July 31st-August 4th 2010, Tours, France. 324 pp
Nyasani JO, Meyhöfer R, Subramanian S & Poehling H.-M (2012). Effect of intercrops on thrips species composition and population abundance on French beans in Kenya. Entomologia Experimentalis et Applicata 142, 236-246
Palmer JM (1990). Identification of the common thrips of Tropical Africa (Thysanoptera, Insecta). Tropical Pest Management. 36 (1): 27-49
Palmer JM, Mound LA & du Heaume GJ (1989). 2. Thysanoptera, 73 pp. In Betts CR [ed.], CIE Guides to insects of importance to man. CAB International, Wallingford, Oxon, UK
Pitkin BR (1978). Lectotype designations of certain species of thrips described by J. D. Hood and notes on his collection (Thysanoptera). Proceedings of the Entomological Society of Washington. 80 (2): 264-295
Pitkin BR & Mound LA (1973). A catalogue of West African Thysanoptera. Bulletin de ľInstitut Fondamental ďAfrique Noire, Sèrie A. 35 (2): 407-449
Priesner H (1964). A monograph of the Thysanoptera of the Egyptian deserts. Publications de lâ Institut du Desert d´Egypte (1960). 13: 1-549
Stannard LJ (1968). The Thrips, or Thysanoptera, of Illinois. Illinois Natural History Survey Bulletin. 29 (4): 215-552
zur Strassen R (1960). Catalogue of the known species of South African Thysanoptera. Journal of the Entomological Society of Southern Africa. 23 (2): 321-367
zur Strassen R (1968). New records of South African Thysanoptera with description of a new Phlaeothripid genus. Journal of the Entomological Society of Southern Africa. 31 (2): 365-372
----
Web links
Mound´s Thysanoptera pages
Thysanoptera Checklist
ICIPE Thrips survey sites
UNI Halle & Thrips sites
Thrips of California